当前位置:
X-MOL 学术
›
Pest Manag. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural optimization of SDH‐targeting chalcone derivatives: piperazine‐driven binding stability against Xanthomonas pathogens
Pest Management Science ( IF 3.8 ) Pub Date : 2025-05-29 , DOI: 10.1002/ps.8934
Tianyu Deng 1 , Kaini Meng 1 , Hong Fu 1 , Yuhong Wang 1 , Hongqian Zou 1 , Ying Yang 1 , Mingman Sun 1 , Lang Xing 1 , Xiujie Yu 1 , Da Liu 2 , Wei Xue 1
Pest Management Science ( IF 3.8 ) Pub Date : 2025-05-29 , DOI: 10.1002/ps.8934
Tianyu Deng 1 , Kaini Meng 1 , Hong Fu 1 , Yuhong Wang 1 , Hongqian Zou 1 , Ying Yang 1 , Mingman Sun 1 , Lang Xing 1 , Xiujie Yu 1 , Da Liu 2 , Wei Xue 1
Affiliation
|
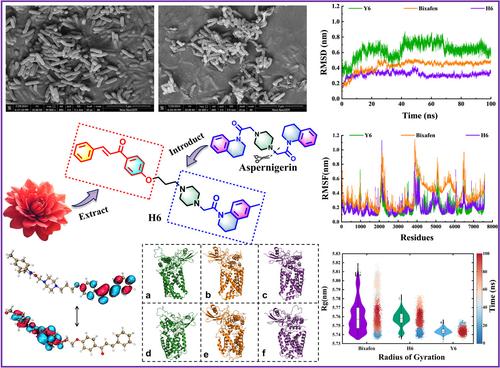
BACKGROUNDNatural green pesticides have become a global research hotspot, and identifying chemical structural frameworks with excellent biological activity has become the research direction of numerous researchers.RESULTSTwenty chalcone derivatives incorporating 1,2,3,4‐tetrahydroquinoline scaffolds were systematically evaluated for their antibacterial activity against six plant pathogenic bacteria. Among the tested compounds, H1–H10 exhibited superior in vitro inhibition against Xanthomonas citri pv. mangiferaeindicae (Xcm ) compared to Y1–Y10. Notably, compound H6 demonstrated exceptional potency, with a median effective concentration (EC50 ) value of 3.25 μg mL−1 against Xcm , significantly surpassing the commercial agent (TC, EC50 = 75.34 μg mL−1 ). In vivo efficacy trials revealed that H6 achieved 65.24% curative activity at 100 μg mL−1 , outperforming TC (42.81%). Scanning electron microscopy further confirmed H6's disruptive effects on bacterial membrane integrity. Mechanistic studies targeting succinate dehydrogenase (SDH), a key respiratory enzyme, revealed structural and energetic similarities between H6 and the commercial SDH inhibitor bixafen through molecular docking and dynamics simulations.CONCLUSIONThe 1,2,3,4‐tetrahydroquinoline moiety enhanced SDH binding affinity, while the introduced piperazine substructure in H6 improved both complex stability (root mean square deviation <1.5 Å) and target engagement. These findings establish H6 as a promising lead compound for developing next‐generation SDH inhibitors, providing critical insights into structure–activity relationships for agricultural antimicrobial design. © 2025 Society of Chemical Industry.
中文翻译:

靶向 SDH 的查尔酮衍生物的结构优化:哌嗪驱动的对黄单胞菌病原体的结合稳定性
背景自然绿色农药已成为全球研究热点,鉴定具有优异生物活性的化学结构框架成为众多研究人员的研究方向。结果系统评价了含有 1,2,3,4-四氢喹啉支架的 20 个查尔酮衍生物对 6 种植物病原菌的抗菌活性。在测试的化合物中,H1-H10 对 Xanthomonas citri pv 表现出优异的体外抑制作用。mangiferaeindicae (Xcm) 与 Y1-Y10 的比较。值得注意的是,化合物 H6 表现出非凡的效力,对 Xcm 的中位有效浓度 (EC50) 值为 3.25 μg mL-1,显著优于商业制剂 (TC,EC50 = 75.34 μg mL-1)。体内疗效试验显示,H6 在 100 μg mL-1 时达到 65.24% 的疗效,优于 TC (42.81%)。扫描电子显微镜进一步证实了 H6 对细菌膜完整性的破坏性影响。针对关键呼吸酶琥珀酸脱氢酶 (SDH) 的机制研究通过分子对接和动力学模拟揭示了 H6 与市售 SDH 抑制剂 bixafen 之间的结构和能量相似性。结论 1,2,3,4-四氢喹啉部分增强了 SDH 结合亲和力,而 H6 中引入的哌嗪亚结构提高了复合物稳定性 (均方根偏差 <1.5 Å) 和靶标结合。这些发现将 H6 确立为开发下一代 SDH 抑制剂的有前途的先导化合物,为农业抗菌设计的构效关系提供了重要见解。© 2025 化工学会.
更新日期:2025-05-29
中文翻译:

靶向 SDH 的查尔酮衍生物的结构优化:哌嗪驱动的对黄单胞菌病原体的结合稳定性
背景自然绿色农药已成为全球研究热点,鉴定具有优异生物活性的化学结构框架成为众多研究人员的研究方向。结果系统评价了含有 1,2,3,4-四氢喹啉支架的 20 个查尔酮衍生物对 6 种植物病原菌的抗菌活性。在测试的化合物中,H1-H10 对 Xanthomonas citri pv 表现出优异的体外抑制作用。mangiferaeindicae (Xcm) 与 Y1-Y10 的比较。值得注意的是,化合物 H6 表现出非凡的效力,对 Xcm 的中位有效浓度 (EC50) 值为 3.25 μg mL-1,显著优于商业制剂 (TC,EC50 = 75.34 μg mL-1)。体内疗效试验显示,H6 在 100 μg mL-1 时达到 65.24% 的疗效,优于 TC (42.81%)。扫描电子显微镜进一步证实了 H6 对细菌膜完整性的破坏性影响。针对关键呼吸酶琥珀酸脱氢酶 (SDH) 的机制研究通过分子对接和动力学模拟揭示了 H6 与市售 SDH 抑制剂 bixafen 之间的结构和能量相似性。结论 1,2,3,4-四氢喹啉部分增强了 SDH 结合亲和力,而 H6 中引入的哌嗪亚结构提高了复合物稳定性 (均方根偏差 <1.5 Å) 和靶标结合。这些发现将 H6 确立为开发下一代 SDH 抑制剂的有前途的先导化合物,为农业抗菌设计的构效关系提供了重要见解。© 2025 化工学会.


















































 京公网安备 11010802027423号
京公网安备 11010802027423号