当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Interplay between Gut Microbiota-Derived Metabolites and Ferroptosis: Implications for Intestinal Health and Disease
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2025-06-02 , DOI: 10.1021/acs.jafc.5c00130
Chenzhe Gao, Jiahui Ma, Yang Yu, Lin Zhang, Yue Pang, Haiyan Zhang, Chenyu Xue, Dehai Li, Xiaoyu Zhao, Munkh-Amgalan Gantumur, Mizhou Hui, Weichen Hong, Yihong Bao, Bailiang Li, Na Dong
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2025-06-02 , DOI: 10.1021/acs.jafc.5c00130
Chenzhe Gao, Jiahui Ma, Yang Yu, Lin Zhang, Yue Pang, Haiyan Zhang, Chenyu Xue, Dehai Li, Xiaoyu Zhao, Munkh-Amgalan Gantumur, Mizhou Hui, Weichen Hong, Yihong Bao, Bailiang Li, Na Dong
|
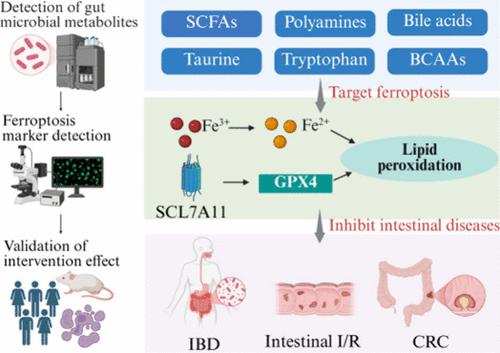
Ferroptosis, an iron-dependent form of regulated cell death driven by lipid peroxidation (LPO), has emerged as a critical player in intestinal health and disease. The gut microbiota, through metabolic activity, generate bioactive metabolites that influence host physiology, including the regulation of ferroptosis. Recent studies have highlighted the pivotal role of gut microbiota-derived metabolites in sustaining intestinal homeostasis. Although the human body has evolved natural mechanisms, such as ferroptosis, to maintain gut equilibrium, elucidating the contribution of gut microbiota-derived metabolites to this process remains a critical area of research. This Review systematically examines the molecular mechanisms underlying ferroptosis, the interplay between gut microbial metabolites and ferroptotic pathways, and the implications for intestinal disorders. Furthermore, it explores innovative therapeutic strategies targeting microbial metabolite–ferroptosis interactions. By synthesizing current evidence, this work aims to advance the development of microbiota-centric therapies for ferroptosis-related intestinal pathologies, bridging mechanistic insights with translational potential.
中文翻译:

肠道微生物群衍生代谢物与铁死亡之间的相互作用:对肠道健康和疾病的影响
铁死亡是一种由脂质过氧化 (LPO) 驱动的铁依赖性调节细胞死亡形式,已成为肠道健康和疾病的关键参与者。肠道微生物群通过代谢活动产生影响宿主生理学的生物活性代谢物,包括铁死亡的调节。最近的研究强调了肠道微生物群衍生代谢物在维持肠道稳态中的关键作用。尽管人体已经进化出自然机制(例如铁死亡)来维持肠道平衡,但阐明肠道微生物群衍生代谢物对这一过程的贡献仍然是一个关键的研究领域。本综述系统地研究了铁死亡的分子机制、肠道微生物代谢物与铁死亡途径之间的相互作用以及对肠道疾病的影响。此外,它还探索了针对微生物代谢物-铁死亡相互作用的创新治疗策略。通过综合当前证据,这项工作旨在推进以微生物群为中心的疗法的开发,用于治疗铁死亡相关的肠道病理,将机制见解与转化潜力联系起来。
更新日期:2025-06-03
中文翻译:

肠道微生物群衍生代谢物与铁死亡之间的相互作用:对肠道健康和疾病的影响
铁死亡是一种由脂质过氧化 (LPO) 驱动的铁依赖性调节细胞死亡形式,已成为肠道健康和疾病的关键参与者。肠道微生物群通过代谢活动产生影响宿主生理学的生物活性代谢物,包括铁死亡的调节。最近的研究强调了肠道微生物群衍生代谢物在维持肠道稳态中的关键作用。尽管人体已经进化出自然机制(例如铁死亡)来维持肠道平衡,但阐明肠道微生物群衍生代谢物对这一过程的贡献仍然是一个关键的研究领域。本综述系统地研究了铁死亡的分子机制、肠道微生物代谢物与铁死亡途径之间的相互作用以及对肠道疾病的影响。此外,它还探索了针对微生物代谢物-铁死亡相互作用的创新治疗策略。通过综合当前证据,这项工作旨在推进以微生物群为中心的疗法的开发,用于治疗铁死亡相关的肠道病理,将机制见解与转化潜力联系起来。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号