当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and Synthesis of Thymol Derivatives Bearing a 1,2,3-Triazole Moiety for Papaya Protection against Fusarium solani
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2025-06-03 , DOI: 10.1021/acs.jafc.4c12770
Mariana Belizário de Oliveira, Poliana Aparecida Rodrigues Gazolla, Leandra Martins Meireles, Róbson Ricardo Teixeira, Danilo Aniceto da Silva, Luiz Claudio Almeida Barbosa, Pedro Alves Bezerra Morais, Osmair Vital de Oliveira, Claudia Jorge do Nascimento, Pedro Henrique de Andrade Barrela, Jochen Junker, Nayara Araujo dos Santos, Wanderson Romão, Valdemar Lacerda, Waldir Cintra de Jesus Júnior, Eduardo Seiti Gomide Mizubuti, Vagner Tebaldi de Queiroz, Demetrius Profeti, Willian Bucker Moraes, Rodrigo Scherer, Adilson Vidal Costa
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2025-06-03 , DOI: 10.1021/acs.jafc.4c12770
Mariana Belizário de Oliveira, Poliana Aparecida Rodrigues Gazolla, Leandra Martins Meireles, Róbson Ricardo Teixeira, Danilo Aniceto da Silva, Luiz Claudio Almeida Barbosa, Pedro Alves Bezerra Morais, Osmair Vital de Oliveira, Claudia Jorge do Nascimento, Pedro Henrique de Andrade Barrela, Jochen Junker, Nayara Araujo dos Santos, Wanderson Romão, Valdemar Lacerda, Waldir Cintra de Jesus Júnior, Eduardo Seiti Gomide Mizubuti, Vagner Tebaldi de Queiroz, Demetrius Profeti, Willian Bucker Moraes, Rodrigo Scherer, Adilson Vidal Costa
|
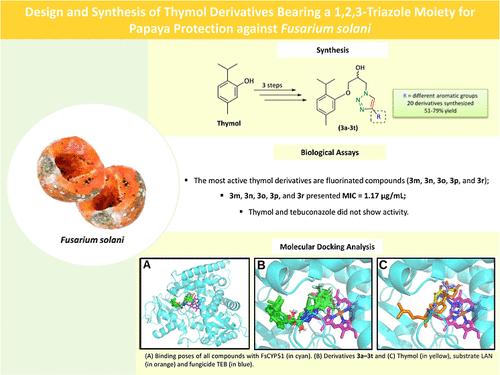
Azole-based fungicides are among the market’s most widely used and effective agents. However, their indiscriminate use can lead to reduced efficacy and increased pathogen resistance. This highlights the need for novel fungicides that offer improved efficiency and lower environmental impact for controlling phytopathogenic fungi. In this study, a series of 20 novel thymol derivatives, incorporating a 1,2,3-triazole moiety, were synthesized via a three-step process, with the key step being the copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction. The antifungal activity of these compounds was evaluated against Fusarium solani, the etiological agent of papaya fruit and stem rot. Additionally, molecular docking was performed to assess the binding energy and interaction modes of these derivatives with the F. solani lanosterol 14α-demethylase (FsCYP51) enzyme. Docking results demonstrated that all derivatives bound to the catalytic pocket of FsCYP51 with lower binding energy (<−10 kcal/mol) compared to the azole fungicide tebuconazole (−8.2 kcal/mol) and the substrate lanosterol (−9.0 kcal/mol). The observed fungicidal activity is likely due to the occupancy of the entrance tunnel and active site of the FsCYP51 by these derivatives, thereby blocking lanosterol and its conversion into ergosterol.
中文翻译:

带有 1,2,3-三唑部分的百里香酚衍生物的设计与合成,用于木瓜对茄属镰刀菌的防护
唑类杀菌剂是市场上使用最广泛和最有效的药剂之一。然而,不加选择地使用它们会导致疗效降低和病原体耐药性增加。这凸显了对新型杀菌剂的需求,这些杀菌剂为控制植物病原真菌提供了更高的效率和更低的环境影响。在这项研究中,通过三步法合成了一系列 20 种新型百里香酚衍生物,其中掺入 1,2,3-三唑部分,其中关键步骤是铜 (I) 催化的叠氮化物-炔烃环加成反应 (CuAAC) 反应。针对木瓜果实和茎腐病的病原体 Fusarium solani 评估了这些化合物的抗真菌活性。此外,进行了分子对接以评估这些衍生物与立枯镰刀菌羊毛甾醇 14α-去甲基化酶 (FsCYP51) 酶的结合能和相互作用模式。对接结果表明,与唑类杀菌剂戊唑醇 (-8.2 kcal/mol) 和底物羊毛甾醇 (-9.0 kcal/mol) 相比,所有结合能低于 FsCYP51 催化口袋的衍生物 (<-10 kcal/mol) 都具有较低的结合能 (%9.0 kcal/mol)。观察到的杀真菌活性可能是由于这些衍生物占据了 FsCYP51 的入口隧道和活性位点,从而阻止了羊毛甾醇及其转化为麦角甾醇。
更新日期:2025-06-03
中文翻译:

带有 1,2,3-三唑部分的百里香酚衍生物的设计与合成,用于木瓜对茄属镰刀菌的防护
唑类杀菌剂是市场上使用最广泛和最有效的药剂之一。然而,不加选择地使用它们会导致疗效降低和病原体耐药性增加。这凸显了对新型杀菌剂的需求,这些杀菌剂为控制植物病原真菌提供了更高的效率和更低的环境影响。在这项研究中,通过三步法合成了一系列 20 种新型百里香酚衍生物,其中掺入 1,2,3-三唑部分,其中关键步骤是铜 (I) 催化的叠氮化物-炔烃环加成反应 (CuAAC) 反应。针对木瓜果实和茎腐病的病原体 Fusarium solani 评估了这些化合物的抗真菌活性。此外,进行了分子对接以评估这些衍生物与立枯镰刀菌羊毛甾醇 14α-去甲基化酶 (FsCYP51) 酶的结合能和相互作用模式。对接结果表明,与唑类杀菌剂戊唑醇 (-8.2 kcal/mol) 和底物羊毛甾醇 (-9.0 kcal/mol) 相比,所有结合能低于 FsCYP51 催化口袋的衍生物 (<-10 kcal/mol) 都具有较低的结合能 (%9.0 kcal/mol)。观察到的杀真菌活性可能是由于这些衍生物占据了 FsCYP51 的入口隧道和活性位点,从而阻止了羊毛甾醇及其转化为麦角甾醇。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号