当前位置:
X-MOL 学术
›
Cell Stem Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preferential tumor targeting of HER2 by iPSC-derived CAR T cells engineered to overcome multiple barriers to solid tumor efficacy
Cell Stem Cell ( IF 19.8 ) Pub Date : 2025-06-04 , DOI: 10.1016/j.stem.2025.05.007
Martin P. Hosking, Soheila Shirinbak, Kyla Omilusik, Shilpi Chandra, Mika K. Kaneko, Angela Gentile, Susumu Yamamoto, Bishwas Shrestha, Joy Grant, Megan Boyett, Demetrio Cardenas, Hannah Keegan, Samad Ibitokou, Carolina Pavon, Takahiro Mizoguchi, Tatsuya Ihara, Daisuke Nakayama, Ramzey Abujarour, Tom T. Lee, Raedun Clarke, Bahram Valamehr
Cell Stem Cell ( IF 19.8 ) Pub Date : 2025-06-04 , DOI: 10.1016/j.stem.2025.05.007
Martin P. Hosking, Soheila Shirinbak, Kyla Omilusik, Shilpi Chandra, Mika K. Kaneko, Angela Gentile, Susumu Yamamoto, Bishwas Shrestha, Joy Grant, Megan Boyett, Demetrio Cardenas, Hannah Keegan, Samad Ibitokou, Carolina Pavon, Takahiro Mizoguchi, Tatsuya Ihara, Daisuke Nakayama, Ramzey Abujarour, Tom T. Lee, Raedun Clarke, Bahram Valamehr
|
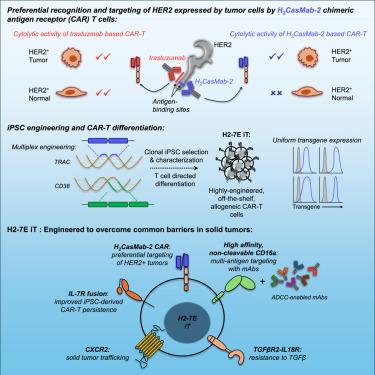
Chimeric antigen receptor (CAR) T cell therapies in solid tumors have been limited by on-target, off-tumor toxicity, antigen heterogeneity, and an inability to simultaneously overcome multiple diverse resistance mechanisms within the tumor microenvironment that attenuate anti-tumor activity. Here, we describe an induced pluripotent stem cell (iPSC)-derived CAR T cell that combines a human epidermal growth factor receptor 2 (HER2)-targeting CAR—differentially recognizing tumor from normal cells and enabling detection of both truncated and misfolded HER2—with multiplex editing designed to address and overcome obstacles to maximize efficacy in solid tumor indications. The iPSC-derived, HER2-directed CAR T cells maintained potent HER2-specific anti-tumor activity in both in vitro and in vivo settings, with limited cytolytic targeting of HER2+ normal targets. Combination with therapeutic antibodies enabled comprehensive multi-antigen targeting through both the CAR and a high-affinity, non-cleavable CD16a Fc receptor. Additionally, specific engineering of interleukin (IL)-7R-fusion, transforming growth factor β (TGF-β)-IL-18R, and CXCR2 enabled sustained persistence, resistance to TGF-β-mediated suppression, and specific migration to the tumor.
中文翻译:

iPSC 来源的 CAR T 细胞优先靶向 HER2 的肿瘤,旨在克服实体瘤疗效的多重障碍
实体瘤中的嵌合抗原受体 (CAR) T 细胞疗法受到靶向、非肿瘤毒性、抗原异质性以及无法同时克服肿瘤微环境中减弱抗肿瘤活性的多种不同耐药机制的限制。在这里,我们描述了一种诱导多能干细胞 (iPSC) 衍生的 CAR T 细胞,它结合了靶向人表皮生长因子受体 2 (HER2) 的 CAR——从正常细胞中区分识别肿瘤并能够检测截短和错误折叠的 HER2——与多重编辑相结合,旨在解决和克服障碍,以最大限度地提高实体瘤适应症的疗效。iPSC 衍生的 HER2 定向 CAR T 细胞在体外和体内均保持有效的 HER2 特异性抗肿瘤活性,对 HER2+ 正常靶标的溶细胞靶向性有限。与治疗性抗体联合使用,可通过 CAR 和高亲和力、不可切割的 CD16a Fc 受体实现全面的多抗原靶向。此外,白细胞介素 (IL)-7R 融合、转化生长因子 β (TGF-β)-IL-18R 和 CXCR2 的特异性工程改造实现了持续持续存在、对 TGF β介导的抑制的耐药性以及向肿瘤的特异性迁移。
更新日期:2025-06-04
中文翻译:

iPSC 来源的 CAR T 细胞优先靶向 HER2 的肿瘤,旨在克服实体瘤疗效的多重障碍
实体瘤中的嵌合抗原受体 (CAR) T 细胞疗法受到靶向、非肿瘤毒性、抗原异质性以及无法同时克服肿瘤微环境中减弱抗肿瘤活性的多种不同耐药机制的限制。在这里,我们描述了一种诱导多能干细胞 (iPSC) 衍生的 CAR T 细胞,它结合了靶向人表皮生长因子受体 2 (HER2) 的 CAR——从正常细胞中区分识别肿瘤并能够检测截短和错误折叠的 HER2——与多重编辑相结合,旨在解决和克服障碍,以最大限度地提高实体瘤适应症的疗效。iPSC 衍生的 HER2 定向 CAR T 细胞在体外和体内均保持有效的 HER2 特异性抗肿瘤活性,对 HER2+ 正常靶标的溶细胞靶向性有限。与治疗性抗体联合使用,可通过 CAR 和高亲和力、不可切割的 CD16a Fc 受体实现全面的多抗原靶向。此外,白细胞介素 (IL)-7R 融合、转化生长因子 β (TGF-β)-IL-18R 和 CXCR2 的特异性工程改造实现了持续持续存在、对 TGF β介导的抑制的耐药性以及向肿瘤的特异性迁移。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号