当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering Red Blood Cells for Amplified Tumor Dual-Gas Transfusion Therapy
Advanced Materials ( IF 27.4 ) Pub Date : 2025-06-04 , DOI: 10.1002/adma.202503206
Tao Li, Min Ouyang, Wei Wang, Sijin Chen, Chengxinqiao Wang, Jiahui Lai, Peixian Weng, Zhenhua Li, Yupeng Wang, Dongfang Zhou
Advanced Materials ( IF 27.4 ) Pub Date : 2025-06-04 , DOI: 10.1002/adma.202503206
Tao Li, Min Ouyang, Wei Wang, Sijin Chen, Chengxinqiao Wang, Jiahui Lai, Peixian Weng, Zhenhua Li, Yupeng Wang, Dongfang Zhou
|
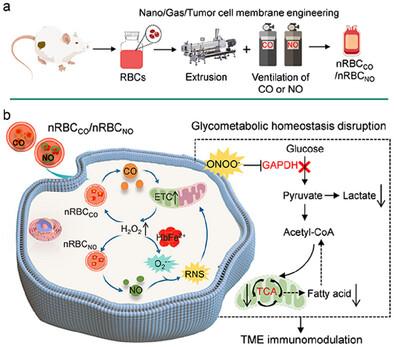
Red blood cell (RBC) transfusion therapy constitutes a vital medical intervention primarily aimed at enhancing oxygen delivery. Furthermore, RBCs possess the ability to stably bind therapeutic gas molecules such as carbon monoxide (CO) and nitric oxide (NO). As natural gas carriers, RBCs have the potential to mitigate the non-specific gas release and biosafety issues associated with conventional gas donors, which currently hinder the clinical application of gas therapy. In this study, RBCs are innovatively engineered for amplified tumor dual-gas transfusion therapy. The RBCs delivering CO and NO are developed using advanced nano- and gas-engineering techniques. These engineered RBCs are activated by tumor cell-specific hydrogen peroxide (H2O2) to release gases, and it induces a cascade amplification of reactive oxygen species (ROS) and reactive nitrogen species (RNS) (ONOO−) production through the catalytic action of the ferrous hemoglobin (HbFe2+). This process disrupts glycometabolism and reshapes the tumor immunosuppressive microenvironment, thereby enhancing therapeutic efficacy. By integrating tumor cell membrane engineering, this approach enables targeted, personalized therapy and effectively suppresses metastatic tumors synergistically with αPD-L1. Comprehensive evaluation demonstrates that engineered RBC-based amplified tumor dual-gas transfusion therapy exhibits excellent biosafety, and holds significant potential as a highly translatable and promising cancer treatment modality.
中文翻译:

用于扩增肿瘤双气体输血治疗的工程红细胞
红细胞 (RBC) 输血疗法是一种重要的医疗干预措施,主要旨在提高氧气输送。此外,红细胞具有稳定结合一氧化碳 (CO) 和一氧化氮 (NO) 等治疗性气体分子的能力。作为天然气载体,红细胞有可能缓解与传统气体供体相关的非特异性气体释放和生物安全问题,这些问题目前阻碍了气体疗法的临床应用。在这项研究中,红细胞被创新地设计为用于扩增的肿瘤双气输血治疗。输送 CO 和 NO 的 RBC 是使用先进的纳米和气体工程技术开发的。这些工程红细胞被肿瘤细胞特异性过氧化氢 (H2O2) 激活以释放气体,并通过亚铁血红蛋白 (HbFe2+) 的催化作用诱导活性氧 (ROS) 和活性氮 (RNS) (ONOO−) 的级联扩增产生。这个过程破坏了糖代谢并重塑了肿瘤免疫抑制微环境,从而提高了治疗效果。通过整合肿瘤细胞膜工程,这种方法可实现靶向、个性化治疗,并与 αPD-L1 协同有效抑制转移性肿瘤。综合评估表明,工程化的基于 RBC 的扩增肿瘤双气输血疗法表现出优异的生物安全性,作为一种高度可转化和有前途的癌症治疗方式具有巨大潜力。
更新日期:2025-06-04
中文翻译:

用于扩增肿瘤双气体输血治疗的工程红细胞
红细胞 (RBC) 输血疗法是一种重要的医疗干预措施,主要旨在提高氧气输送。此外,红细胞具有稳定结合一氧化碳 (CO) 和一氧化氮 (NO) 等治疗性气体分子的能力。作为天然气载体,红细胞有可能缓解与传统气体供体相关的非特异性气体释放和生物安全问题,这些问题目前阻碍了气体疗法的临床应用。在这项研究中,红细胞被创新地设计为用于扩增的肿瘤双气输血治疗。输送 CO 和 NO 的 RBC 是使用先进的纳米和气体工程技术开发的。这些工程红细胞被肿瘤细胞特异性过氧化氢 (H2O2) 激活以释放气体,并通过亚铁血红蛋白 (HbFe2+) 的催化作用诱导活性氧 (ROS) 和活性氮 (RNS) (ONOO−) 的级联扩增产生。这个过程破坏了糖代谢并重塑了肿瘤免疫抑制微环境,从而提高了治疗效果。通过整合肿瘤细胞膜工程,这种方法可实现靶向、个性化治疗,并与 αPD-L1 协同有效抑制转移性肿瘤。综合评估表明,工程化的基于 RBC 的扩增肿瘤双气输血疗法表现出优异的生物安全性,作为一种高度可转化和有前途的癌症治疗方式具有巨大潜力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号