当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improving the Soluble Expression of Sweet Protein Thaumatin II through Directed Evolution in Escherichia coli
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2025-06-03 , DOI: 10.1021/acs.jafc.5c01078
Lefei Wang, Zilong Liu, Liling Zhang, Yuxin Liu, Xinyao Li, Liying Zhu, Zhengming Zhu, Ling Jiang
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2025-06-03 , DOI: 10.1021/acs.jafc.5c01078
Lefei Wang, Zilong Liu, Liling Zhang, Yuxin Liu, Xinyao Li, Liying Zhu, Zhengming Zhu, Ling Jiang
|
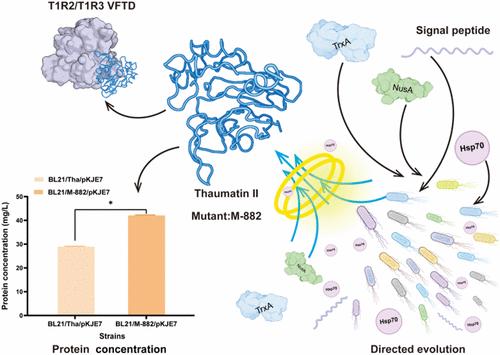
Thaumatin is a natural sweet protein valued for its intense sweetness, long-lasting effect, low-calorie content, and safety. However, limited solubility and low yields hinder microbial fermentation. To overcome these challenges, we engineered Escherichia coli to express thaumatin II and implemented three strategies to improve its solubility. Coexpression with molecular chaperones proved to be the most effective in significantly enhancing the soluble expression of thaumatin II. Furthermore, we applied directed evolution to further refine thaumatin II, resulting in the M-882 mutant, which achieved a thaumatin titer of 42 mg/L, representing a 45% increase compared to the original protein yield. Structural analysis revealed that disruption of two adjacent disulfide bonds in the M-882 mutant minimized mispairing during peptide folding, thereby improving protein solubility. Additionally, molecular dynamics simulations showed that the M-882 variant enhanced solvent accessibility and structural flexibility, both of which contributed to improved solubility. The interaction between thaumatin II and sweet taste receptors, as well as the analysis of surface positive charges, indicated that the M-882 variant is capable of binding to the receptors, thereby retaining its sweetness. Overall, this study not only offers valuable insights into optimizing thaumatin expression but also establishes a foundation for engineering other sweet proteins with broad industrial applications.
中文翻译:

通过大肠杆菌中的定向进化改善甜蛋白索马甜 II 的可溶性表达
Thaumatin 是一种天然甜味蛋白,因其强烈的甜味、持久的效果、低热量含量和安全性而受到重视。然而,有限的溶解度和低产量阻碍了微生物发酵。为了克服这些挑战,我们设计了大肠杆菌来表达奇马甜 II,并实施了三种策略来提高其溶解度。与分子伴侣共表达被证明在显着增强奇马汀 II 的可溶性表达方面最有效。此外,我们应用定向进化进一步精炼奇马甜 II,得到 M-882 突变体,其奇马甜滴度达到 42 mg/L,与原始蛋白产量相比增加了 45%。结构分析显示,M-882 突变体中两个相邻二硫键的破坏最大限度地减少了肽折叠过程中的错配,从而提高了蛋白质溶解度。此外,分子动力学模拟表明,M-882 变体增强了溶剂的可及性和结构灵活性,这两者都有助于提高溶解度。奇马汀 II 与甜味受体之间的相互作用,以及表面正电荷的分析表明,M-882 变体能够与受体结合,从而保持其甜味。总体而言,这项研究不仅为优化奇马甜表达提供了有价值的见解,还为工程化其他具有广泛工业应用的甜味蛋白奠定了基础。
更新日期:2025-06-04
中文翻译:

通过大肠杆菌中的定向进化改善甜蛋白索马甜 II 的可溶性表达
Thaumatin 是一种天然甜味蛋白,因其强烈的甜味、持久的效果、低热量含量和安全性而受到重视。然而,有限的溶解度和低产量阻碍了微生物发酵。为了克服这些挑战,我们设计了大肠杆菌来表达奇马甜 II,并实施了三种策略来提高其溶解度。与分子伴侣共表达被证明在显着增强奇马汀 II 的可溶性表达方面最有效。此外,我们应用定向进化进一步精炼奇马甜 II,得到 M-882 突变体,其奇马甜滴度达到 42 mg/L,与原始蛋白产量相比增加了 45%。结构分析显示,M-882 突变体中两个相邻二硫键的破坏最大限度地减少了肽折叠过程中的错配,从而提高了蛋白质溶解度。此外,分子动力学模拟表明,M-882 变体增强了溶剂的可及性和结构灵活性,这两者都有助于提高溶解度。奇马汀 II 与甜味受体之间的相互作用,以及表面正电荷的分析表明,M-882 变体能够与受体结合,从而保持其甜味。总体而言,这项研究不仅为优化奇马甜表达提供了有价值的见解,还为工程化其他具有广泛工业应用的甜味蛋白奠定了基础。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号