当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Novel Pyrazole β-Ketonitrile Derivatives as Broad Spectrum SDHI Fungicides by Introducing a Flexible Motif
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2025-06-03 , DOI: 10.1021/acs.jafc.4c12476
Liangliang Cheng, Hanting Wang, Cong Zhou, Jiawei Qin, Zhong Li, Jiagao Cheng
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2025-06-03 , DOI: 10.1021/acs.jafc.4c12476
Liangliang Cheng, Hanting Wang, Cong Zhou, Jiawei Qin, Zhong Li, Jiagao Cheng
|
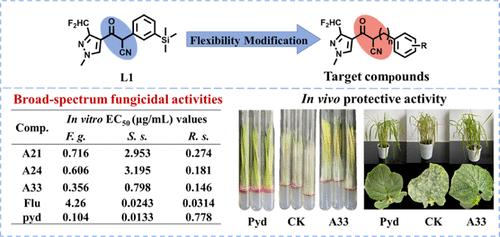
Scaffold optimization plays a critical role in the exploration of fungicides with improved potency and an expanded spectrum. In this study, a series of novel pyrazole β-ketonitrile derivatives were rationally designed and identified as potent broad-spectrum succinate dehydrogenase inhibitor fungicides, through modifying the molecular flexibility via a flexible methylene motif. After stepwise modifications, compounds A21, A24, and A33 displayed a broad spectrum of antifungal activities in vitro against Fusarium graminearum, Sclerotinia sclerotiorum, and Rhizoctonia solani. Especially, compound A33 (EC50 = 0.356 μg/mL) exhibited comparable in vitro fungicidal activities against F. graminearum to the positive control pydiflumetofen (EC50 = 0.104 μg/mL). In the greenhouse assay, compound A33 displayed moderate in vivo protective effects against rice blast at 100 μg/mL, significant in vivo protection against cucumber powdery mildew at 50 μg/mL, and moderate in vivo protective effects against wheat scab at 200 μg/mL. The succinate dehydrogenase (SDH) inhibitory assay revealed that A21, A24, and A33 are potent SDH inhibitors with inhibitory concentration values of 0.123, 0.0317, and 0.0709 μM, respectively. Docking results demonstrated that H-bonds and cation–π interactions between A33 and residues of Trp173, Tyr91, and Arg46 are important for the binding of compounds within SDH. Our findings revealed that the improvement of flexibility in the pyrazole β-ketonitrile scaffold is an effective approach to explore novel potent broad-spectrum SDHIs.
中文翻译:

通过引入柔性基序发现新型吡唑 β-酮腈衍生物作为广谱 SDHI 杀菌剂
支架优化在探索具有更高效力和扩展谱的杀菌剂中起着关键作用。本研究通过柔性亚甲基基序修饰分子柔韧性,合理设计了一系列新型吡唑 β-酮腈衍生物,鉴定为有效的广谱琥珀酸脱氢酶抑制剂杀菌剂。经过逐步修饰,化合物 A21、A24 和 A33 在体外对禾谷镰刀菌 、菌核菌和立枯丝核菌表现出广谱的抗真菌活性。特别是,化合物 A33 (EC50 = 0.356 μg/mL) 对禾谷镰刀菌的体外杀菌活性与阳性对照吡地氟氧芬 (EC50 = 0.104 μg/mL) 相当。在温室试验中,化合物 A33 在 100 μg/mL 时对稻瘟病表现出中等体内保护作用,在 50 μg/mL 时对黄瓜白粉病表现出显著的体内保护作用,在 200 μg/mL 时对小麦赤霉病表现出中等体内保护作用。琥珀酸脱氢酶 (SDH) 抑制试验显示 A21、A24 和 A33 是有效的 SDH 抑制剂,抑制浓度值分别为 0.123、0.0317 和 0.0709 μM。对接结果表明,A33 与 Trp173、Tyr91 和 Arg46 残基之间的 H 键和阳离子-π相互作用对 SDH 内化合物的结合很重要。我们的研究结果表明,吡唑 β-酮腈支架柔韧性的提高是探索新型有效广谱 SDHIs 的有效方法。
更新日期:2025-06-04
中文翻译:

通过引入柔性基序发现新型吡唑 β-酮腈衍生物作为广谱 SDHI 杀菌剂
支架优化在探索具有更高效力和扩展谱的杀菌剂中起着关键作用。本研究通过柔性亚甲基基序修饰分子柔韧性,合理设计了一系列新型吡唑 β-酮腈衍生物,鉴定为有效的广谱琥珀酸脱氢酶抑制剂杀菌剂。经过逐步修饰,化合物 A21、A24 和 A33 在体外对禾谷镰刀菌 、菌核菌和立枯丝核菌表现出广谱的抗真菌活性。特别是,化合物 A33 (EC50 = 0.356 μg/mL) 对禾谷镰刀菌的体外杀菌活性与阳性对照吡地氟氧芬 (EC50 = 0.104 μg/mL) 相当。在温室试验中,化合物 A33 在 100 μg/mL 时对稻瘟病表现出中等体内保护作用,在 50 μg/mL 时对黄瓜白粉病表现出显著的体内保护作用,在 200 μg/mL 时对小麦赤霉病表现出中等体内保护作用。琥珀酸脱氢酶 (SDH) 抑制试验显示 A21、A24 和 A33 是有效的 SDH 抑制剂,抑制浓度值分别为 0.123、0.0317 和 0.0709 μM。对接结果表明,A33 与 Trp173、Tyr91 和 Arg46 残基之间的 H 键和阳离子-π相互作用对 SDH 内化合物的结合很重要。我们的研究结果表明,吡唑 β-酮腈支架柔韧性的提高是探索新型有效广谱 SDHIs 的有效方法。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号