当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Click-Controlled Photo-Uncaging: Click-Assembly of Platinum-Based Photoactive Protecting Groups for Light-Triggered Bioactive Molecule Release
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2025-06-03 , DOI: 10.1021/jacs.5c02579
Arpit Sharma, Wjdan Jogadi, Man Kshetri, Suha Alqarni, Md Al Amin, May Cheline, Bishal Pokhrel, Shirin Akter, Zexin Lin, Jimin Park, Jordan Solmen, Jordan Caraway, Megan Brattley, Hao Shen, Yao-Rong Zheng
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2025-06-03 , DOI: 10.1021/jacs.5c02579
Arpit Sharma, Wjdan Jogadi, Man Kshetri, Suha Alqarni, Md Al Amin, May Cheline, Bishal Pokhrel, Shirin Akter, Zexin Lin, Jimin Park, Jordan Solmen, Jordan Caraway, Megan Brattley, Hao Shen, Yao-Rong Zheng
|
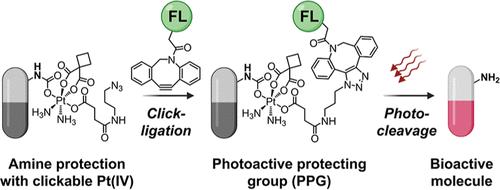
We introduce click-controlled photouncaging, an innovative approach that synergizes click ligation with photocleavage to achieve biorthogonal, light-triggered bioactive molecule release across visible to near-infrared (NIR) wavelengths. Central to this approach is a novel amine protection and deprotection strategy utilizing Pt(IV) complexes. In this strategy, an azide-bearing clickable Pt(IV) moiety acts as a protecting group for amine-containing molecules (the ″cargo″) via a carbamate linkage. Subsequently, click ligation with a DBCO-tagged fluorescent antenna positions the antenna near the Pt(IV) core, transforming it into a photoactive protecting group (PPG) that can be fine-tuned to respond to visible or NIR light. When exposed to light, the antenna drives the photoreduction of the Pt(IV) linker, triggering deprotection and subsequently releasing the cargo molecule. To validate this approach, three Pt(IV) complexes were synthesized featuring amine-containing fluorescent reporters (coumarin, BODIPY) and a therapeutic molecule (Exatecan), and their functionality was validated in solution and cell cultures. Overall, this work introduces a novel, user-friendly, and versatile chemical tool for bioorthogonal, light-controlled activation of molecules in complex biological environments.
中文翻译:

点击控制光解笼:铂基光活性保护基团的点击组装,用于光触发生物活性分子释放
我们介绍了点击控制光解锁,这是一种创新方法,可将点击连接与光切割协同,以实现在可见光到近红外 (NIR) 波长范围内的双正交、光触发的生物活性分子释放。这种方法的核心是利用 Pt(IV) 配合物的新型胺保护和脱保护策略。在这种策略中,带有叠氮化物的可点击 Pt(IV)部分通过氨基甲酸酯键充当含胺分子(“货物”)的保护基团。随后,使用 DBCO 标记的荧光天线进行点击连接,将天线定位在 Pt(IV) 核心附近,将其转化为光活性保护基团 (PPG),可以对其进行微调以响应可见光或 NIR 光。当暴露在光线下时,天线驱动 Pt(IV) 接头的光还原,触发脱保护并随后释放货物分子。为了验证这种方法,合成了三种 Pt(IV) 复合物,具有含胺的荧光报告基因(香豆素、BODIPY)和一种治疗分子 (Exatecan),并在溶液和细胞培养物中验证了它们的功能。总体而言,这项工作介绍了一种新颖、用户友好且用途广泛的化学工具,用于在复杂生物环境中对分子进行生物正交、光控激活。
更新日期:2025-06-04
中文翻译:

点击控制光解笼:铂基光活性保护基团的点击组装,用于光触发生物活性分子释放
我们介绍了点击控制光解锁,这是一种创新方法,可将点击连接与光切割协同,以实现在可见光到近红外 (NIR) 波长范围内的双正交、光触发的生物活性分子释放。这种方法的核心是利用 Pt(IV) 配合物的新型胺保护和脱保护策略。在这种策略中,带有叠氮化物的可点击 Pt(IV)部分通过氨基甲酸酯键充当含胺分子(“货物”)的保护基团。随后,使用 DBCO 标记的荧光天线进行点击连接,将天线定位在 Pt(IV) 核心附近,将其转化为光活性保护基团 (PPG),可以对其进行微调以响应可见光或 NIR 光。当暴露在光线下时,天线驱动 Pt(IV) 接头的光还原,触发脱保护并随后释放货物分子。为了验证这种方法,合成了三种 Pt(IV) 复合物,具有含胺的荧光报告基因(香豆素、BODIPY)和一种治疗分子 (Exatecan),并在溶液和细胞培养物中验证了它们的功能。总体而言,这项工作介绍了一种新颖、用户友好且用途广泛的化学工具,用于在复杂生物环境中对分子进行生物正交、光控激活。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号