当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Breaking the Scaling Relationship in Water Oxidation Enabled by the Electron Buffering Effect of the Fullerene Network
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2025-06-04 , DOI: 10.1021/jacs.5c03577
Xiang Chen, Hao Ma, Xing Wang, Hongqiang Jin, Yao Wu, Sibo Wang, Yukun Xiao, Rui Jiang, Yumin Da, Lei Fan, Yuanmiao Sun, Shibo Xi, Yanwei Lum, Qian He, Hexing Li, Dongming Liu, Shangfeng Yang, Wei Chen
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2025-06-04 , DOI: 10.1021/jacs.5c03577
Xiang Chen, Hao Ma, Xing Wang, Hongqiang Jin, Yao Wu, Sibo Wang, Yukun Xiao, Rui Jiang, Yumin Da, Lei Fan, Yuanmiao Sun, Shibo Xi, Yanwei Lum, Qian He, Hexing Li, Dongming Liu, Shangfeng Yang, Wei Chen
|
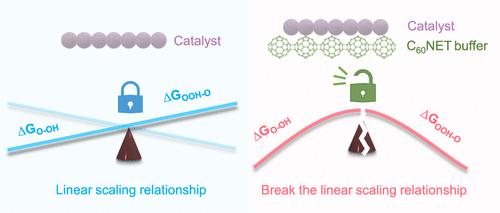
The scaling relationship among reaction intermediates with strongly correlated adsorption energy in the oxygen evolution reaction (OER) severely restricts the energy-conversion efficiency of water electrolysis. For the conventional adsorbate evolution mechanism, breaking the scaling relationship remains challenging, as it is difficult to modulate the adsorption of multiple intermediates on a specific active site simultaneously. Herein, we utilize the electron buffering effect of a two-dimensional fullerene network (C60NET) to dynamically tune the electronic structure of the iridium (Ir) active site with the change of adsorbed intermediates, which can tailor the adsorption strength of intermediates from multistep reactions and break the adsorption-energy scaling relationships among *OOH, *O, and *OH. The C60NET-buffered Ir nanocluster catalyst exhibits excellent OER activity with a low overpotential of 237 mV and stability over 600 h at 10 mA cm–2, outperforming graphene-supported Ir nanoclusters and commercial IrOx, attributed to the breaking of the linear scaling relationship enabled by the unique ability to reversibly accept and donate electrons of C60NET.
中文翻译:

打破富勒烯网络的电子缓冲效应在水氧化中的结垢关系
在析氧反应 (OER) 中,吸附能强相关的反应中间体之间的结垢关系严重制约了电解水的能量转换效率。对于传统的吸附物析出机制,打破缩放关系仍然具有挑战性,因为很难同时调节多个中间体在特定活性位点上的吸附。在此,我们利用二维富勒烯网络 (C60NET) 的电子缓冲效应,随着吸附中间体的变化动态调节铱 (Ir) 活性位点的电子结构,这可以从多步反应中定制中间体的吸附强度,并打破 *OOH、*O 和 *OH 之间的吸附-能量缩放关系。C60NET 缓冲 Ir 纳米团簇催化剂表现出优异的 OER 活性,具有 237 mV 的低过电位,在 10 mA cm–2 下超过 600 小时的稳定性,优于石墨烯负载的 Ir 纳米团簇和商业 IrOx,这归因于打破了线性缩放关系,这归因于可逆接受和提供 C60NET 电子的独特能力所实现的线性缩放关系。
更新日期:2025-06-04
中文翻译:

打破富勒烯网络的电子缓冲效应在水氧化中的结垢关系
在析氧反应 (OER) 中,吸附能强相关的反应中间体之间的结垢关系严重制约了电解水的能量转换效率。对于传统的吸附物析出机制,打破缩放关系仍然具有挑战性,因为很难同时调节多个中间体在特定活性位点上的吸附。在此,我们利用二维富勒烯网络 (C60NET) 的电子缓冲效应,随着吸附中间体的变化动态调节铱 (Ir) 活性位点的电子结构,这可以从多步反应中定制中间体的吸附强度,并打破 *OOH、*O 和 *OH 之间的吸附-能量缩放关系。C60NET 缓冲 Ir 纳米团簇催化剂表现出优异的 OER 活性,具有 237 mV 的低过电位,在 10 mA cm–2 下超过 600 小时的稳定性,优于石墨烯负载的 Ir 纳米团簇和商业 IrOx,这归因于打破了线性缩放关系,这归因于可逆接受和提供 C60NET 电子的独特能力所实现的线性缩放关系。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号