当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reinvestigation of the C-Glycoside Synthase in Alnumycin Biosynthesis Reveals a Conserved Mechanism of C–C Bond Formation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2025-06-04 , DOI: 10.1021/jacs.5c03469
Daan Ren, Yu-Hsuan Lee, Hung-wen Liu
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2025-06-04 , DOI: 10.1021/jacs.5c03469
Daan Ren, Yu-Hsuan Lee, Hung-wen Liu
|
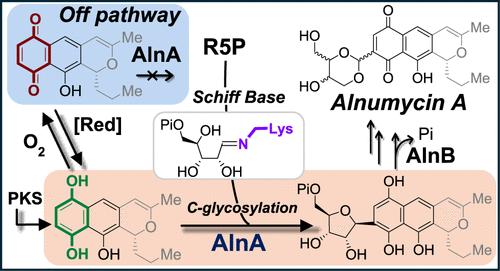
C-Nucleosides are defined by their unusual C–C glycosidic linkage between the nucleobase and the monosaccharide moiety, which distinguishes them from common N-nucleosides. Several enzymes have been identified to catalyze this atypical C–C bond formation. For instance, YeiN catalyzes the reversible cleavage of pseudouridine 5′-phosphate, yielding ribose 5-phosphate (R5P) and uracil via a Schiff base intermediate formed between R5P and an active-site lysine residue. In alnumycin biosynthesis, the C–C glycosidic bond between R5P and a naphthoquinone heterocycle, prealnumycin, has been shown to be installed by AlnA. While AlnA shares 41% sequence identity with YeiN, a distinct mechanism involving an ene-diol tautomer of R5P had been proposed based on previous biochemical studies and X-ray crystallography. Herein, the mechanism of AlnA is reevaluated using juglone (5-hydroxy-1,4-naphthalenedione) as a prealnumycin analog. By employing isotopologues and protein mass spectrometry, the involvement of an ene-diol intermediate and an alternative Morita–Baylis–Hillman mechanism in AlnA catalysis can both be ruled out. Further analysis of juglone reactivity showed that it can be reduced either enzymatically when coupled to glucose oxidase or nonenzymatically through autoreduction yielding 1,4,5-naphthalenetriol. This hydroquinone derivative of juglone serves as the true substrate of AlnA such that the C-glycosylation mechanism is no different from that of YeiN. These findings unravel the correct substrate of the C-glycoside synthase AlnA and unify the mechanisms of AlnA, YeiN, and other C-glycoside synthases. These results highlight that accurate substrate identification is essential for mechanistic study of enzyme catalysis and call for a reevaluation of the biosynthetic pathway of alnumycin and other naphthoquinone-derived natural products.
中文翻译:

重新研究阿努霉素生物合成中的 C-糖苷合酶揭示了 C-C 键形成的保守机制
C-核苷由核碱基和单糖部分之间不寻常的 C-C 糖苷键定义,这使它们与常见的 N-核苷区分开来。已经确定了几种酶来催化这种非典型的 C-C 键形成。例如,YeiN 催化假尿苷 5′-磷酸的可逆裂解,通过 R5P 和活性位点赖氨酸残基之间形成的席夫碱中间体产生 5-磷酸核糖 (R5P) 和尿嘧啶。在 alnumycin 生物合成中,R5P 和萘醌杂环 prealnumycin 之间的 C-C 糖苷键已被证明是由 AlnA 安装的。虽然 AlnA 与 YeiN 具有 41% 的序列同一性,但基于以前的生化研究和 X 射线晶体学,已经提出了一种涉及 R5P 烯二醇互变异构体的独特机制。在此,使用 juglone (5-hydroxy-1,4-naphthalenedione) 作为 prealnumycin 类似物重新评估 AlnA 的机制。通过采用同位素体和蛋白质质谱法,可以排除烯-二醇中间体和替代 Morita-Baylis-Hillman 机制参与 AlnA 催化的可能性。对 Juglone 反应性的进一步分析表明,当它与葡萄糖氧化酶偶联时,它可以被酶促降低,或者通过自还原产生 1,4,5-萘三醇而被非酶促降低。这种 juglone 的对苯二酚衍生物是 AlnA 的真正底物,因此 C-糖基化机制与 YeiN 没有什么不同。这些发现揭示了 C-糖苷合酶 AlnA 的正确底物,并统一了 AlnA、YeiN 和其他 C-糖苷合酶的机制。 这些结果强调,准确的底物鉴定对于酶催化的机制研究至关重要,并呼吁重新评估阿纳霉素和其他萘醌衍生天然产物的生物合成途径。
更新日期:2025-06-04
中文翻译:

重新研究阿努霉素生物合成中的 C-糖苷合酶揭示了 C-C 键形成的保守机制
C-核苷由核碱基和单糖部分之间不寻常的 C-C 糖苷键定义,这使它们与常见的 N-核苷区分开来。已经确定了几种酶来催化这种非典型的 C-C 键形成。例如,YeiN 催化假尿苷 5′-磷酸的可逆裂解,通过 R5P 和活性位点赖氨酸残基之间形成的席夫碱中间体产生 5-磷酸核糖 (R5P) 和尿嘧啶。在 alnumycin 生物合成中,R5P 和萘醌杂环 prealnumycin 之间的 C-C 糖苷键已被证明是由 AlnA 安装的。虽然 AlnA 与 YeiN 具有 41% 的序列同一性,但基于以前的生化研究和 X 射线晶体学,已经提出了一种涉及 R5P 烯二醇互变异构体的独特机制。在此,使用 juglone (5-hydroxy-1,4-naphthalenedione) 作为 prealnumycin 类似物重新评估 AlnA 的机制。通过采用同位素体和蛋白质质谱法,可以排除烯-二醇中间体和替代 Morita-Baylis-Hillman 机制参与 AlnA 催化的可能性。对 Juglone 反应性的进一步分析表明,当它与葡萄糖氧化酶偶联时,它可以被酶促降低,或者通过自还原产生 1,4,5-萘三醇而被非酶促降低。这种 juglone 的对苯二酚衍生物是 AlnA 的真正底物,因此 C-糖基化机制与 YeiN 没有什么不同。这些发现揭示了 C-糖苷合酶 AlnA 的正确底物,并统一了 AlnA、YeiN 和其他 C-糖苷合酶的机制。 这些结果强调,准确的底物鉴定对于酶催化的机制研究至关重要,并呼吁重新评估阿纳霉素和其他萘醌衍生天然产物的生物合成途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号